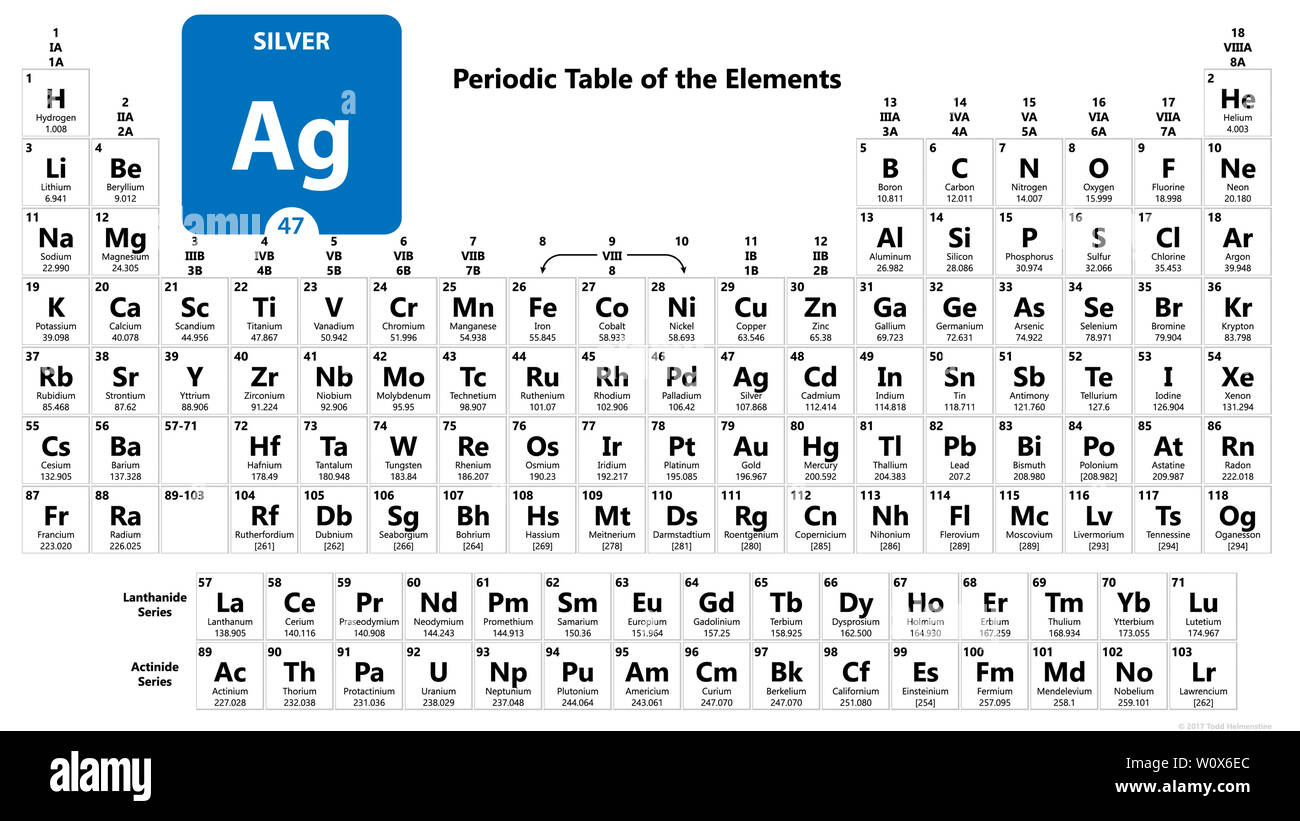
Silver Periodic Table
A vertical column in the periodic table.
Silver periodic table. Silver periodic table. It is located in period 5 and group 11. The atomic number of each element increases by one reading from left to right. It has 47 protons and 47 electrons in the atomic structure.
These blocks are named. This places it in the middle of the second full row period of the table. Chemical element in the periodic table of elements. Cus 2 agno 3 aq cuno 3 2 2 ag s.
Members of a group typically have similar properties and electron configurations in their outer shell. Silver was predominantly present in form of ores and various techniques were developed as early as in the 4 th millennium bc to extract pure silver. Silver is readily available commercially so it is not normally necessary to prepare silver in the laboratoryhowever the formation of silver metal may be demonstrated in a satisfying reaction in which copper metal is dipped into a solution of silver nitrate agno 3. Silver is similar in its physical and chemical properties to its two vertical neighbours in group 11 of the periodic table copper and goldits 47 electrons are arranged in the configuration kr4d 10 5s 1 similarly to copper ar3d 10 4s 1 and gold xe4f 14 5d 10 6s 1.
The result is formation of often attractive. Period a horizontal row in the periodic table. The chemical symbol for silver is ag. Silver is a 47.
Block elements are organised into blocks by the orbital type in which the outer electrons are found. Later romans became the biggest dealers of silvers and their. Greeks and romans also used silver as currency.